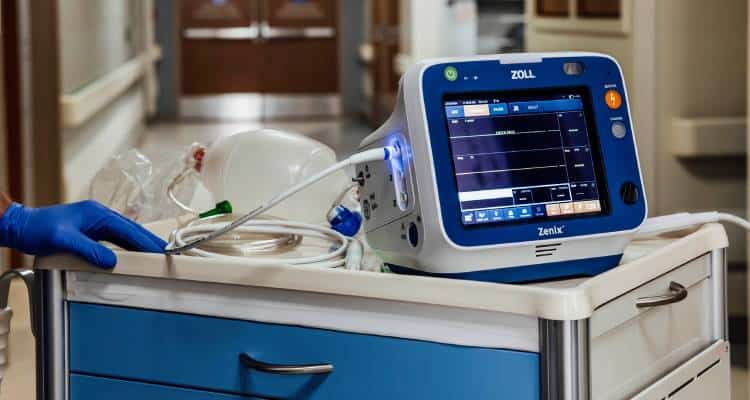
The Zenix monitor/defibrillator is designed for use in EMS and hospital settings
Asahi Kasei has advanced its suite of critical care products through premarket approval by the U.S. Food and Drug Administration (FDA) for ZOLL’s Zenix monitor/defibrillator. The Zenix device is the culmination of years of user feedback and is ZOLL’s most clinically advanced, user-friendly professional monitor/defibrillator. Asahi Kasei’s critical care lineup under ZOLL is a key growth driver supporting long-term corporate development.
The Zenix monitor/defibrillator is designed for use in EMS and hospital settings. It features a large, durable touchscreen and functionality to automate workflows. Users can dynamically customize the device with real-time adjustments that make it easy to use in critical moments. The device also features ZOLL’s Real BVM Help and exclusive Real CPR Help® technology. ZOLL has been a major source of growth for Asahi Kasei’s Healthcare sector, driving a fivefold increase in net sales and a sevenfold rise in operating income since the company joined the Asahi Kasei Group in 2012. In its medium-term management plan, Asahi Kasei has indicated it will capitalise on this steady growth by introducing new products while maintaining market competitiveness. The Zenix monitor/defibrillator is a prime example of how the company delivers high-value products based on a sophisticated understanding of customer needs.
“The approval of Zenix reflects our strategic, long-term focus on growth through high-value, solution-based technologies. This device has been designed with an understanding of customer needs, and we expect it will be well-received by customers and healthcare professionals,” commented Ken Shinomiya, Leader of the Healthcare Sector at Asahi Kasei. “This innovation expands our critical care portfolio and offers healthcare providers an excellent tool to tackle their key challenges. We will stay competitive and reinforce our commitment to long-term profitable growth through these innovations.”