– A minimally invasive treatment for individuals suffering from loss of smell and taste could become a reality. Led by Thomas Jefferson University Hospital otolaryngologist Dr. David Rosen, a team of physicians and researchers have developed a first-of-its-kind topical platelet-rich plasma treatment. Preliminary results from an ongoing clinical trial show promise in restoring patients’ sense of smell and taste.
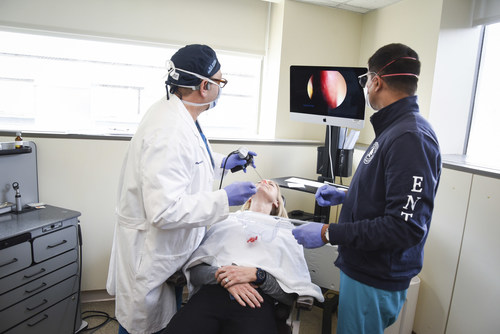
Smell and taste disturbances known as anosmia and parosmia have grown in awareness in recent years since it is a common symptom of COVID-19. While the symptoms typically resolve for most individuals, up to 1.5 million people in the United States continue to experience long-term distortion of the sense of smell and taste.
Platelet-rich plasma (PRP) is a common restorative therapy used to regenerate cells, heal tissue, and address an array of medical conditions from healing injured muscles and tendons to increasing hair growth and reducing the appearance of scars. Animal studies have shown that PRP helps regenerate the olfactory epithelium, which may be the site affected in COVID-19 induced olfactory dysfunction (OD). As smell and taste are closely interrelated, improved sense of smell can help with sense of taste as well. Until now, PRP has been used as a nasal injectable in several small clinical trials for smell loss. Although the results were promising, nasal injections can be uncomfortable and invasive for patients.
“I’ve dedicated over two decades to helping patients recover from the loss of taste and smell,” said Dr. David Rosen, MD, Otolaryngologist, Thomas Jefferson University Hospital. “It was very important to me and our team to explore less invasive options as this issue has become increasingly prevalent due to COVID-19. The results of phase I of the clinical trial have been promising and we are looking forward to phase II to further improve the treatment.”
The new topical PRP treatment consists of monthly applications for a minimum of three months. A recent phase I clinical trial of eight patients who had at least six months of olfactory disturbance has shown preliminary success with 50 percent of participants experiencing clinically significant improvements in smell and taste. While phase I only consisted of eight patients, it is the largest pilot study to date for the use of PRP in treatment of OD and the first study to develop methods for topical delivery in human subjects. The new treatment has also been provided to dozens of additional patients independent of the phase I clinical trial with promising results.
A planned phase II study aims to exclusively look at patients who developed long term olfactory disturbance following recovery from COVID-19 infection. This will help the research team better understand patient variables and the number of treatments required to maintain sustainable improvements in smell and taste.
“Thomas Jefferson University is at the forefront of cutting-edge research to advance science and medical treatments around the globe,” said Dr. Mark L. Tykocinski, Executive Vice President for Academic Affairs and Provost, Thomas Jefferson University. “I thank this team of researchers and patient volunteers who are helping to create this important breakthrough and hopefully improve the quality-of-life for countless people experiencing this unfortunate long-COVID symptom.”
Nancy A. Damato, Thomas Jefferson University Hospital patient and participant in the clinical trial added, “Losing my smell and taste from COVID has been life changing. I felt like I was missing a part of myself and more than anything I missed the experience of gathering with family to enjoy a meal. Fortunately, the treatments provided by Thomas Jefferson University Hospital are improving my symptoms and showing signs of progress. For the first time in a long time, I have hope for getting my life back to normal.”
“The widespread smell dysfunction that accompanied the COVID-19 virus has raised awareness of the many ways in which smell loss impacts health and well-being on a daily basis,” said Pamela Dalton, PhD, MPH, Member, Monell Chemical Senses Center. “The promising results from this study bring hopeful news to the many individuals who continue to experience persistent post-viral smell loss.”
The treatment is currently not covered by insurance and costs $500 per application but will be available to patients enrolled in the next phase of the clinical study free of charge once it begins. Individuals interested in learning more about this treatment or who wish to schedule a consultation can call 1-800-JEFF-NOW (1-800-533-3669), email [email protected], or visit JeffersonHealth.org/otolaryngology.
Photos of patients receiving treatment are available HERE (© Thomas Jefferson University Photography Services.)
About Jefferson Health
Jefferson Health, home of Sidney Kimmel Medical College, is reimagining health care in the greater Philadelphia region and southern New Jersey. Jefferson’s dedicated team of doctors, nurses, health professionals and staff provide a range of primary to highly-specialized care through 18 hospitals (ten are Magnet®-designated by the ANCC for nursing excellence), more than 50 outpatient and urgent care locations, the NCI-designated Sidney Kimmel Cancer Center, MossRehab, Magee Rehabilitation and the JeffConnect® telemedicine program. For 2021-2022, Thomas Jefferson University Hospitals is ranked among the nation’s best in six specialties by U.S. News & World Report. Jefferson Health is also home to Health Partners Plan, a not-for-profit managed healthcare organization serving more than 290,000 members in Southeastern Pennsylvania with a broad range of health coverage options through Health Partners Medicare, Health Partners (Medicaid) and KidzPartners (Children’s Health Insurance Program). Jefferson Health’s mission is to improve the lives of patients in the communities it is privileged to serve through safe, effective, equitable and compassionate care.
About Thomas Jefferson University
Thomas Jefferson University is a leader in transdisciplinary, professional education. Jefferson, home of the Sidney Kimmel Medical College and the Kanbar College of Design, Engineering and Commerce, is a preeminent university delivering high-impact education in 160 undergraduate and graduate programs to 8,400 students in architecture, business, design, engineering, fashion and textiles, health, medicine, science and social science. Jefferson is re-defining the higher education value proposition with an approach that is collaborative and active; increasingly global; integrated with industry; focused on research across disciplines to foster innovation and discovery; and technology-enhanced. Student-athletes compete as the Jefferson Rams in the NCAA Division II Central Atlantic Collegiate Conference.
SOURCE Thomas Jefferson University