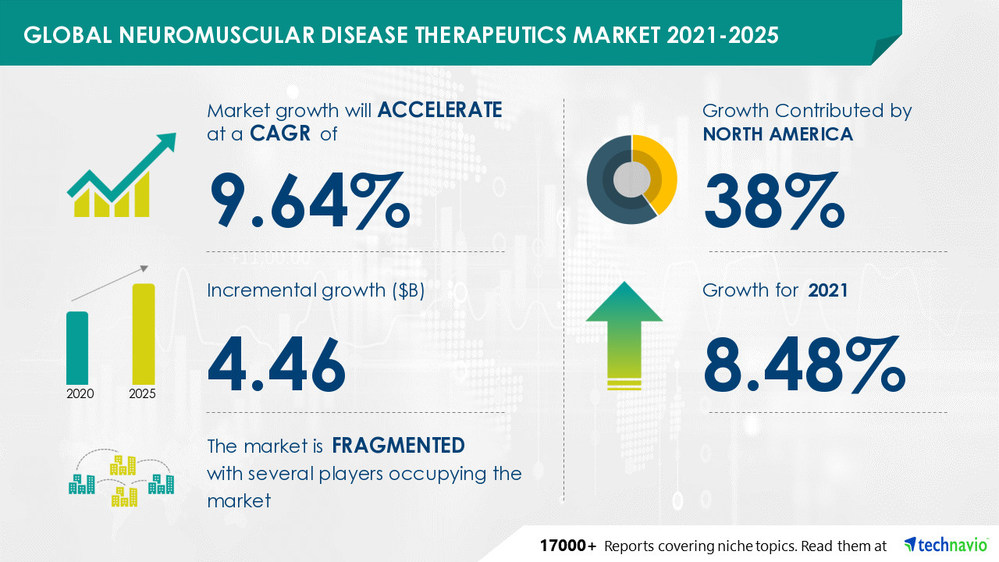
The neuromuscular disease therapeutics market is expected to grow by USD 4.46 bn from 2020 to 2025. The growth momentum of the market will accelerate at a CAGR of 9.64% during the forecast period.
Find additional highlights related to the market. Download Free Sample Report
Neuromuscular Disease Therapeutics Market: Driver
The novel approvals is one of the key factors driving the growth of the neuromuscular disease therapeutics market. There has been an increase in drug approvals in the market for treating various neuromuscular indications in recent years. This is due to the advanced research being conducted on highly morbid and progressive indications. Spinal muscular atrophy is one of the major neuromuscular diseases for which the market has witnessed therapeutic approvals. Spinal muscular atrophy is morbid and rapidly progressive neuromuscular. It is caused by the death of a type of nerve cells, called motor neurons, in the spinal cord. Treatments using drugs are not effective and do not stop the disease progression due to the rapidly progressive nature of the disease. To overcome this challenge, various vendors are conducting research on the development of novel gene therapies and antisense therapies that can stop the progression of the disease and increase the life expectancy of patients.
Learn about more drivers impacting the growth of the market. Request Our Free Sample
Neuromuscular Disease Therapeutics Market: Some Key Vendors and their Offerings
- AFM-Telethon – The company offers GNT 0004 that is based on an adeno-associated virus capsid and an optimized gene.
- Biogen Inc. – The company offers SPINRAZA, which is a prescription medicine used to treat spinal muscular atrophy in pediatric and adult patients.
- Brainstorm Cell Therapeutics Inc. – The company offers MSC-NTF cells that are safe and well-tolerated, with the majority of adverse events being mild or moderate in severity reduction in the rate of ALS disease progression.
- F. Hoffmann-La Roche Ltd. – The company offers Evrysdi, which is used for the treatment of spinal muscular atrophy in adults and children two months of age and older.
- Mitsubishi Chemical Holdings Corp. – The company offers efficacy and safety of FTY720 in patients with relapsing multiple sclerosis.
Get lifetime access to our Technavio Insights. Subscribe now to our most popular “Lite Plan” billed annually at USD 3000. View 3 reports monthly and Download 3 Reports Annually!
Neuromuscular Disease Therapeutics Market: Segmentation Analysis
By type, the neuromuscular disease therapeutics market has been segmented by biologics and small molecules. The biologics segment will have significant market share growth during the forecast period. SPINRAZA by Biogen and ZOLGENSMA by Novartis will dominate this segment.
By geography, the neuromuscular disease therapeutics market has been segmented by North America, Europe, Asia, and ROW. North America will have significant market share growth during the forecast period. The increasing prevalence of neuromuscular diseases and the availability of highly advanced therapeutics for the treatment of these diseases will facilitate the neuromuscular disease therapeutics market growth in North America over the forecast period.
Know more about the global trends impacting the future of the market, Download Free Sample Report
Related Reports:
Radiopharmaceuticals Market by Application and Geography – Forecast and Analysis 2022-2026
| Neuromuscular Disease Therapeutics Market Scope | |
| Report Coverage | Details |
| Page number | 120 |
| Base year | 2020 |
| Forecast period | 2021-2025 |
| Growth momentum & CAGR | Accelerate at a CAGR of 9.64% |
| Market growth 2021-2025 | USD 4.46 billion |
| Market structure | Fragmented |
| YoY growth (%) | 8.48 |
| Regional analysis | North America, Europe, Asia, and ROW |
| Performing market contribution | North America at 38% |
| Key consumer countries | US, Canada, Germany, UK, and China |
| Competitive landscape | Leading companies, Competitive strategies, Consumer engagement scope |
| Key companies profiled | AFM-Telethon, Biogen Inc., Brainstorm Cell Therapeutics Inc., F. Hoffmann-La Roche Ltd., Mitsubishi Chemical Holdings Corp., Novartis AG, Pfizer Inc., PTC Therapeutics Inc., Sanofi SA, and Sarepta Therapeutics Inc. |
| Market dynamics | Parent market analysis, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID-19 impact and recovery analysis and future consumer dynamics, Market condition analysis for the forecast period |
| Customization purview | If our report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
Table of Contents
1. Executive Summary
2. Market Landscape
2.1 Market ecosystem
2.1.1 Parent Market
Exhibit 01: Parent market
Exhibit 02: Market characteristics
2.2 Value chain analysis
Exhibit 03: Value chain analysis: Pharmaceuticals
2.2.1 Research and development (R&D) and drug discovery
2.2.2 Integration and product development
2.2.3 Manufacturing
2.2.4 Outbound logistics
2.2.5 Marketing and sales
2.2.6 Support services
2.2.7 Industry innovations
3. Market Sizing
3.1 Market definition
Exhibit 04: Offerings of vendors included in the market definition
3.2 Market segment analysis
Exhibit 05: Market segments
3.3 Market size 2020
3.4 Market outlook: Forecast for 2020 – 2025
3.4.1 Estimating growth rates for emerging and high-growth markets
3.4.2 Estimating growth rates for mature markets
Exhibit 06: Global – Market size and forecast 2020 – 2025 ($ million)
Exhibit 07: Global market: Year-over-year growth 2020 – 2025 (%)
4. Five Forces Analysis
4.1 Five Forces Summary
Exhibit 08: Five forces analysis 2020 & 2025
4.2 Bargaining power of buyers
Exhibit 09: Bargaining power of buyers
4.3 Bargaining power of suppliers
Exhibit 10: Bargaining power of suppliers
4.4 Threat of new entrants
Exhibit 11: Threat of new entrants
4.5 Threat of substitutes
Exhibit 12: Threat of substitutes
4.6 Threat of rivalry
Exhibit 13: Threat of rivalry
4.7 Market condition
Exhibit 14: Market condition – Five forces 2020
5. Market Segmentation by Type
5.1 Market segments
The segments covered in this chapter are:
- Biologics
- Small molecules
Exhibit 15: Type – Market share 2020-2025 (%)
5.2 Comparison by Type
Exhibit 16: Comparison by Type
5.3 Biologics – Market size and forecast 2020-2025
Exhibit 17: Biologics – Market size and forecast 2020-2025 ($ million)
Exhibit 18: Biologics – Year-over-year growth 2020-2025 (%)
5.4 Small molecules – Market size and forecast 2020-2025
Exhibit 19: Small molecules – Market size and forecast 2020-2025 ($ million)
Exhibit 20: Small molecules – Year-over-year growth 2020-2025 (%)
5.5 Market opportunity by Type
Exhibit 21: Market opportunity by Type
6. Customer landscape
Technavio’s customer landscape matrix comparing Drivers or price sensitivity, Adoption lifecycle, importance in customer price basket, Adoption rate and Key purchase criteria
Exhibit 22: Customer landscape
7. Geographic Landscape
7.1 Geographic segmentation
The regions covered in the report are:
- North America
- Europe
- Asia
- ROW
Exhibit 23: Market share by geography 2020-2025 (%)
7.2 Geographic comparison
Exhibit 24: Geographic comparison
7.3 North America – Market size and forecast 2020-2025
Exhibit 25: North America – Market size and forecast 2020-2025 ($ million)
Exhibit 26: North America – Year-over-year growth 2020-2025 (%)
7.4 Europe – Market size and forecast 2020-2025
Exhibit 27: Europe – Market size and forecast 2020-2025 ($ million)
Exhibit 28: Europe – Year-over-year growth 2020-2025 (%)
7.5 Asia – Market size and forecast 2020-2025
Exhibit 29: Asia – Market size and forecast 2020-2025 ($ million)
Exhibit 30: Asia – Year-over-year growth 2020-2025 (%)
7.6 ROW – Market size and forecast 2020-2025
Exhibit 31: ROW – Market size and forecast 2020-2025 ($ million)
Exhibit 32: ROW – Year-over-year growth 2020-2025 (%)
7.7 Key leading countries
Exhibit 33: Key leading countries
7.8 Market opportunity by geography
Exhibit 34: Market opportunity by geography ($ million)
8. Drivers, Challenges, and Trends
8.1 Market drivers
8.1.1 Novel approvals
8.1.2 Newborn screening tests
8.1.3 Huge unmet need for neuromuscular diseases therapeutics
8.2 Market challenges
8.2.1 Economic burden on patients
8.2.3 Fast progressive nature of neuromuscular diseases
8.2.3 Expiry of patents on major therapeutics
Exhibit 35: Impact of drivers and challenges
8.3 Market trends
8.3.1 Technological advances
8.3.2 Inherited nature of neuromuscular diseases
8.3.3 Increasing research funding
9. Vendor Landscape
9.1 Vendor landscape
Exhibit 36: Vendor landscape
9.2 Landscape disruption
Exhibit 37: Landscape disruption
Exhibit 38: Industry risks
9.3 Competitive Scenario
10. Vendor Analysis
10.1 Vendors covered
Exhibit 39: Vendors covered
10.2 Market positioning of vendors
Exhibit 40: Market positioning of vendors
10.3 AFM-Telethon
Exhibit 41: AFM-Telethon – Overview
Exhibit 42: AFM-Telethon – Product and service
Exhibit 43: AFM-Telethon – Key offerings
10.4 Biogen Inc.
Exhibit 44: Biogen Inc. – Overview
Exhibit 45: Biogen Inc. – Business segments
Exhibit 46: Biogen Inc. – Key offerings
Exhibit 47: Biogen Inc. – Segment focus
10.5 Brainstorm Cell Therapeutics Inc.
Exhibit 48: Brainstorm Cell Therapeutics Inc. – Overview
Exhibit 49: Brainstorm Cell Therapeutics Inc. – Product and service
Exhibit 50: Brainstorm Cell Therapeutics Inc. – Key news
Exhibit 51: Brainstorm Cell Therapeutics Inc. – Key offerings
10.6 F. Hoffmann-La Roche Ltd.
Exhibit 52: F. Hoffmann-La Roche Ltd. – Overview
Exhibit 53: F. Hoffmann-La Roche Ltd. – Business segments
Exhibit 54: F. Hoffmann-La Roche Ltd. – Key offerings
Exhibit 55: F. Hoffmann-La Roche Ltd. – Segment focus
10.7 Mitsubishi Chemical Holdings Corp.
Exhibit 56: Mitsubishi Chemical Holdings Corp. – Overview
Exhibit 57: Mitsubishi Chemical Holdings Corp. – Business segments
Exhibit 58: Mitsubishi Chemical Holdings Corp. – Key offerings
Exhibit 59: Mitsubishi Chemical Holdings Corp. – Segment focus
10.8 Novartis AG
Exhibit 60: Novartis AG – Overview
Exhibit 61: Novartis AG – Business segments
Exhibit 62: Novartis AG – Key offerings
Exhibit 63: Novartis AG – Segment focus
10.9 Pfizer Inc.
Exhibit 64: Pfizer Inc. – Overview
Exhibit 65: Pfizer Inc. – Business segments
Exhibit 66: Pfizer Inc. – Key offerings
Exhibit 67: Pfizer Inc. – Segment focus
10.10 PTC Therapeutics Inc.
Exhibit 68: PTC Therapeutics Inc. – Overview
Exhibit 69: PTC Therapeutics Inc. – Business segments
Exhibit 70: PTC Therapeutics Inc. – Key offerings
Exhibit 71: PTC Therapeutics Inc. – Segment focus
10.11 Sanofi SA
Exhibit 72: Sanofi SA – Overview
Exhibit 73: Sanofi SA – Business segments
Exhibit 74: Sanofi SA – Key offerings
Exhibit 75: Sanofi SA – Segment focus
10.12 Sarepta Therapeutics Inc.
Exhibit 76: Sarepta Therapeutics Inc. – Overview
Exhibit 77: Sarepta Therapeutics Inc. – Business segments
Exhibit 78: Sarepta Therapeutics Inc. – Key offerings
Exhibit 79: Sarepta Therapeutics Inc. – Segment focus
11. Appendix
11.1 Scope of the report
11.1.1 Market definition
11.1.2 Objectives
11.1.3 Notes and caveats
11.2 Currency conversion rates for US$
Exhibit 80: Currency conversion rates for US$
11.3 Research Methodology
Exhibit 81: Research Methodology
Exhibit 82: Validation techniques employed for market sizing
Exhibit 83: Information sources
11.4 List of abbreviations
Exhibit 84: List of abbreviations
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provide actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website: www.technavio.com/
SOURCE Technavio